Printer-friendly .pdf version of this factsheet in English
Printer-friendly .pdf version of this factsheet in Spanish
Pathogen
Rhizoctonia crown and root rot is caused by the fungus, Rhizoctonia solani. This fungus is split into 13 different sub-species called ‘Anastomosis Groups (AG 1-13) based on compatible hyphal fusion reaction. AG 2-2 IIIB and IV, AG 3, AG 4 and AG5 can infect table beets, among which AG 2-2 IIB and IV, and AG 4 are predominant groups in table beet growing areas in New York.
Host
The host range of R. solani is broad and can vary between different AGs. The R. solani AGs infecting table beets can also infect sugar beet, soybeans, common beans, turf grass, potatoes, corn, and other vegetables.
Significance
Rhizoctonia crown and root rot can reduce the appeal of produce for fresh market sale. Seedling death (damping off) can significantly reduce the plant population in the field leading to considerable yield loss. Severe disease in mature beets can also significantly reduce the yield and make roots unsuitable for processing. Rhizoctonia root rot can also reduce the storage life of the crop by causing post-harvest decay.
Symptoms
The pathogen can cause both pre- and post-emergence damping off. Post-emergence damping off begins with water soaking of the hypocotyl on the infected seedlings followed by dark brown to black sunken lesions. Lesions can expand to develop a constricted wire-like area on the stem, which becomes brittle and breaks off (Fig. 1).

Infection of mature roots begins with a small brown scarring on the outer surface which eventually develops into active brown to black lesions (Fig. 2 left) that extends inside and outside the root (Fig. 2 right). Cankers and cracks with gray fungal mycelia may also be observed (Fig. 2 middle). Affected roots may also be colonized by secondary bacteria and fungi.

Crown rot starts at the base of the petiole of the older leaves either through direct infection of the petiole in contact with the soil or upward movement of the pathogen from the infected roots. The infected area in the petiole becomes necrotic and leads to stunted foliage and wilting (Fig. 3).

Foliar symptoms are more noticeable during the day in low moisture conditions. Severe disease leads to browning of the foliage and leaf death that gives the plant a spider-like or rosette appearance (Fig. 4).

Disease Cycle
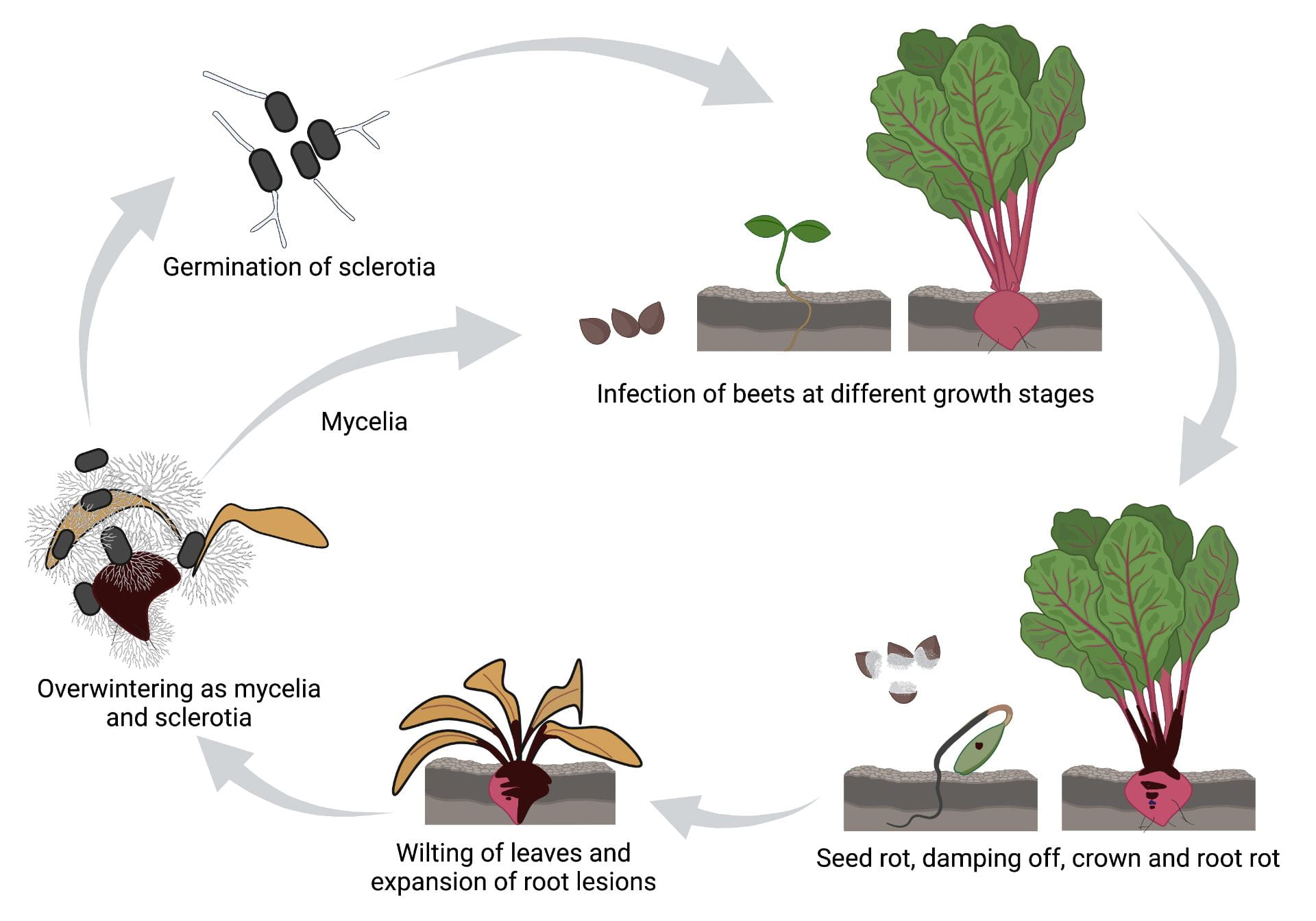
R. solani can survive in plant debris as saprophytic (living on dead or decayed organic matter) mycelia, or sclerotia. Sclerotia are the dark, hardened masses of fungal mycelia that can survive in the soil for more than 3 years. R. solani can infect the table beet plants across a broad range of temperatures (12-35 oC or 53.6-95 oF) and soil moisture (>25%) conditions, but warm temperatures (20-30 oC or 68-86 oF) and high soil moisture (>75%) are highly conducive for disease development. In late spring or early summer, when the soil begins to warm, the sclerotia germinate to develop hyphae which are guided to the plant by chemicals released from the roots (Fig. 5). Once attached to the host surface, R. solani can enter the host via wound, natural openings like stomata, or direct penetration of the host epidermis. Infection is followed by symptoms in the seed, seedling, crown and roots. R. solani does not produce asexual spores, therefore the disease has only one infection cycle within a season. Production of sexual spores that would be responsible for long-distance dispersal, are rare. This disease is therefore disseminated to new locations by infested soil and crop debris, irrigation water, soil erosion, and farm equipment.
Management
Management of Rhizoctonia crown and root rot involves multiple tools to limit the survival of overwintering sclerotia and reduce mycelial growth and infection of seedlings and roots to minimize the carry-over of inoculum in soil between susceptible crops.
Cultural practices
- Crop rotation: Planting beets directly after snap beans or soybeans should be avoided. Corn, other legumes, and vegetable crops can also be alternative hosts, so non-hosts like small grains should be used in rotation. A 3-year rotation is a minimum and longer rotations are preferred, especially in consideration of the broad host range of R. solani.
- Deep tillage: Infested crop residues and sclerotia may be buried below the root zone to enhance degradation and hence reduce the carry-over of inoculum between seasons.
- Early and shallow planting: Early planting may avoid damping off due to warm and humid conditions that favor infection and disease.
- Drainage and soil structure: Improve soil aeration and drainage conditions to reduce fungal growth and disease development.
- Soil movement: Rhizoctonia crown rot can be caused by infested soil thrown onto plants during hilling. Hill at a suitable speed to avoid soil being thrown into the crowns. Infested soil can be carried by equipment between fields. Consider cleaning equipment between fields.
Cultivar selection
- Certain table beet cultivars may provide a level of resistance to infection by R. solani, particularly in mature plants. Please refer to the resistance information provided by seed companies.
Chemical controls
- Conventional Fungicides: In-furrow fungicide applications, such as azoxystrobin (Fungicide Resistant Action Committee (FRAC) 11), can provide acceptable early to mid-season protection. An additional post-emergent banded application may be made at the 4- to 8-leaf stage, but early applications have been shown to be most crucial for disease control. Resistance to azoxystrobin within NY populations of R. solani has not been detected.
- Organic Options: Alternatives to conventional fungicides for control of R. solani exist, but efficacy can vary. Again, early applications that can protect seedlings are best. Ongoing trials at Cornell AgriTech are currently seeking to identify the most effective organic products and application timings and doses.
More information:
Dr. Sarah J. Pethybridge (sjp277@cornell.edu)
Cornell AgriTech
211 Barton Laboratory
Geneva, New York
(315) 744-5359 (cell)
Dr. Julie R. Kikkert (jrk2@cornell.edu)
Cornell Cooperative Extension
Cornell Vegetable Program
Canandaigua, New York
(585) 394-3977 x 404 (office)
Prepared by Pratibha Sharma, Eric Branch, Julie Kikkert, and Sarah Pethybridge (October 2021). Spanish translation by Natalia Pineros.


