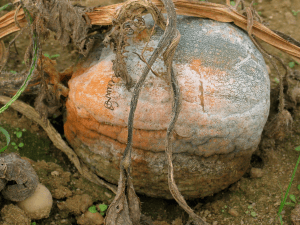Updated July, 2020. (Originally prepared for Vegetable MD Online May 2001.)
This factsheet contains information on the following:
Additional Information:
- Fungicides for Managing Phytophthora Blight in Cucurbits and Other Vegetables
- Mobile Fungicides for Managing Three Major Cucurbit Diseases: Powdery Mildew, Downy Mildew, and Phytophthora Blight
- What Have We Learned About Managing Phytophthora Blight in Cucurbit Crops?
- Results from research conducted at LIHREC on managing Phytophthora blight
Phytophthora blight is a disease that should be a concern to all cucurbit growers. It has been described as the “most destructive disease of cucurbits” because “nothing causes greater loss.” Total crop loss is possible, as in this pumpkin field (Fig. 1, below).
While all cucurbits are susceptible, squash, cucumber and pumpkin seem to be affected more commonly than cantaloupe. It has been increasing in importance in the U.S. Fruit rot was first reported in Colorado and California in the late 1930’s. The disease was found sporadically in most of the U.S. until the 1980’s, except in California, where it occurred more regularly. Then incidence increased notably in Florida, Georgia, North Carolina, New Jersey, Michigan, and the Northeastern U.S. The increase followed a hurricane in some areas. Phytophthora blight also commonly affects peppers and less commonly affects eggplant and tomato.
View symptoms on: peppers | eggplants | tomatoes | beans
Symptoms and Signs
The pathogen, Phytophthora capsici, causes seedling damping-off, leaf spots, foliar blight, root and crown rot, stem lesions, and fruit rot.
Fig. 2: Leaf spots are an uncommon symptom of Phytophthora blight, possibly reflecting fungicide use. They often resemble those caused by Phytophthora infestans, the late blight fungus, being dark brown and typically large with a border of wilted light green tissue which is where the pathogen is progressing as it infects. Initially leaf spots can be mostly wilted light green tissue with little or no necrotic tissue. Leaf spots occur when pathogen spores are splashed onto leaves and when leaf edge is in contact with contaminated soil.
Fig. 3: Phytophthora blight often starts in summer squashes with die back of the growing tip. Infected tissue initially appears water-soaked. Growing tip can be blighted in pumpkin and other vining crops.
Fig. 4: Symptoms of Phytophthora blight in summer squash can progress rapidly from death of the growing tip to total plant collapse and fruit rot.
Fig. 5: Symptoms can also begin at the plant crown. Crown rot causes the entire plant to completely collapse and die in a short period of time. Affected vine tissue initially appears water-soaked, turns brown, and often collapses. Sometimes pathogen sporangia are visible on affected tissue (second image below), and can be induced to form by putting affected plant tissue on wet paper towel in a closed plastic bag over night. Seeing Phytophthora capsici sporangia confirms this pathogen caused the crown rot.
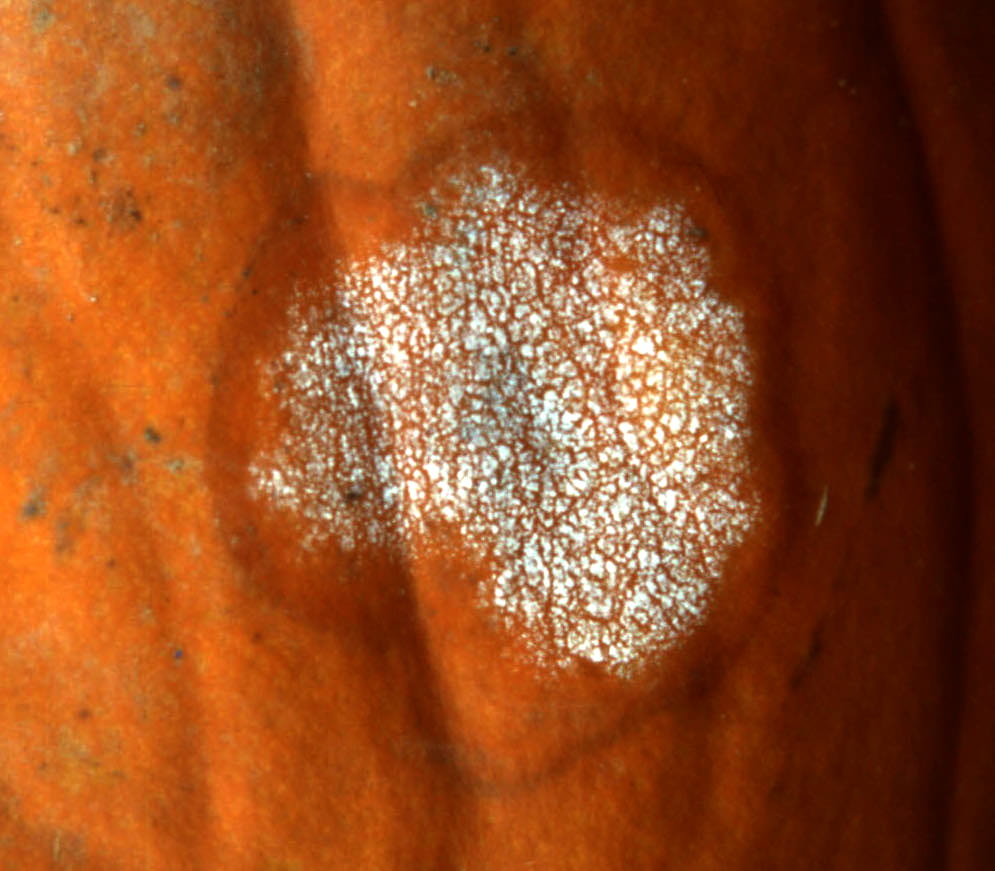
Fig. 6: Phytophthora fruit rot typically starts on the underside of the fruit that is in contact with the ground. This makes it difficult to control with protectant fungicides. The white, yeast-like growth is mostly spores of Phytophthora capsici (sporangia). Seeing sporangia is diagnostic.
Fig. 7: Sometimes the initial symptom is water-soaked lesions on the upper surface of fruit that is the result of pathogen dispersal in splashing water. Lesions are soft and easily punctured when handled. Below are fruit of yellow summer squash and Blue Hubbard squash.
Fig. 8: The pathogen usually produces a white yeast-like growth that contains many sporangia, especially under moist conditions. There are thousands of microscopic, lemon-shaped sporangia on an affected fruit that can be splash dispersed to healthy tissue, which is why development of Phytophthora blight can be explosive. Sporangia either germinate directly and grow into healthy tissue, or several zoospores form inside each sporangium. Zoospores are released from sporangia in water and are able to swim for hours using their two flagella. They are capable of directional movement to a host based on chemical attraction. Sporangia and zoospores are asexual spores, which means they are produced by an individual.
Fig. 9: Less commonly, Phytophthora fruit rot appears as a depressed spot.
Fig. 10: Symptoms can also begin around the stem due to systemic infection from the vine.
Fig. 11: Fruit can become covered with sporangia. Below are fruit of pumpkin and spaghetti squash.
Fig. 12: Sometimes there is no sporulation on affected fruit.
Fig. 13: Saprophytic fungi can grow on tissue killed by Phytophthora capsici causing the fruit to appear to be affected by another disease.
Fig. 14: Fruit can become completely affected and collapse.
Fig. 15: Fruit symptoms also can develop rapidly after harvest.
Two diseases sometimes confused with Phytophthora fruit rot:
Disease Cycle
Phytophthora capsici survives in soil between crops for more than two years. The pathogen can survive longer if thick-walled oospores are produced. Oospores are formed when mycelia of two individuals of opposite mating type (similar to male and female) grow together. Both mating types have been found in some fields, including fields in the northeastern U.S. Since oospores are the product of sexual reproduction (genetic recombination), they are the main source of new races or biotypes, including fungicide resistant biotypes.
Soil moisture conditions are important for disease initiation. Sporangia form when soil is at field capacity (within 24 hours under controlled conditions) and they release zoospores when soil is saturated (5 to 6 hours under controlled conditions). Further disease development in a crop can occur rapidly because sporangia are produced abundantly on affected fruit and they are dispersed under a wider range of conditions than zoospores. Ideal conditions for infection are wet soils above 65° F and air temperatures in the 75 to 85° F range.
Management Practices
Current recommendations center around preventing the pathogen from being moved into a new field and managing soil moisture to avoid saturated conditions which favor disease onset. It is important to use an integrated program with as many of the following practices as possible.
Long rotations between susceptible crops and selecting fields where Phytophthora blight has never occurred seem like important management practices considering this disease is caused by a soil-borne pathogen that does not easily move between fields. However, there have been cases where these were not successful. This may be because:
1. Both mating types of the pathogen are present enabling the pathogen to produce oospores, which are capable of long-term survival.
2. The pathogen can infect roots of some weeds including purslane (see image below).
3. The pathogen can be moved between fields in soil on farm equipment or in running water. Additionally, beneficial antagonistic soil microbes might play a role in suppressing the pathogen following years of Phytophthora blight occurring. However, lack of rotation when targeted fungicides are used could lead to resistance developing sooner on a farm than when fields are rotated. Manage resistance by using a diversity of chemistry, based on FRAC code, in alternation and combination as part of a disease management program that includes cultural practices described below.

- Grow a mustard biofumigant cover crop before a crop susceptible to Phytophthora blight, preferably during spring rather than previous fall.
- Use deep-zone reduced tillage to produce pumpkin, winter squash, or another crop like corn grown in rotation.
- Select well-drained fields.
- Make sure water will be able to drain out of the field. Use a land plane to level the field as much as possible. If water does not normally drain out of the field, then make a trench between beds or rows at their ends, make a ditch or waterway across the end of the field for water coming out of the field in the trenches, and continually grade soil at the end to allow water to leave.
- Physically separate plantings of susceptible crops (cucurbits, pepper, eggplant, and tomato). Plantings should be located such that there is no opportunity for water to move between plantings. The pathogen can also be dispersed in rain splash during storms. Therefore it is prudent to consider prevailing wind direction when deciding where to locate multiple plantings of susceptible crops.
- When growing small-fruited pumpkins, select varieties producing hard, gourd-like rinds (such as Lil’ Ironsides). Mature fruit of such varieties are substantially less susceptible than varieties with conventional rinds which are softer. See research results.
- Minimize hardpans and plowpans by subsoiling or chisel plowing before planting.
- Do not plant the crop in areas of the field with poor drainage. Plant a cover crop in place of the crop in these areas. Image below shows pumpkin crop lost to Phytophthora blight after it started in wet area of field and spread throughout; consequently the grower is disking up the field (right side of image).

- Prepare raised dome-shaped beds for summer squash and other bush-type crops. Ideally beds should be a minimum of 9 inches high. Use a bed shaper to provide more lasting beds as opposed to a simple ridge. Use a transplanter that doesn’t leave a depression around the base of the plant. Fill in any depressions. Raised beds are not recommended for vining crops as some fruit will develop in the low area between beds where conditions will be favorable for Phytophthora blight.
- Covering raised and flat beds with plastic mulch provides a good barrier between crop and pathogen in the soil, but there remains potential for the pathogen to be splash dispersed onto the plastic from soil between mulch strips. This can be especially problematic with heavy fruited vine crops because they can create a depression in the plastic where water can pool creating favorable conditions for infection (see image below).

- With vining crops like pumpkin, it is preferable to plan driveways before seeding and leave them unplanted or seed to something like annual ryegrass (see image below), instead of seeding the entire field and then driving over plants when applying pesticides. Initial symptoms of Phytophthora blight have been seen on pumpkin plants in driveways reflecting compaction from driving through the field creating favorable conditions. Additionally, driving over affected plants is an excellent way to spread the pathogen.

- Minimize hardpans and plowpans by not driving through wet fields.
- Clean farm equipment, shoes, etc. of soil between fields. Movement of contaminated soil on equipment and shoes is likely an important means by which Phytophthora has been spread between fields on farms and may account for the occurrence of Phytophthora blight in fields with no previous history of susceptible crops.
-

Subsoiling between rows after planting and before vining can improve drainage. Subsoil between rows after planting and before vining to improve drainage. Subsoil again as needed after rain. Good drainage is also important for driveways in fields, as symptoms have been observed first on plants next to the compacted soil of driveways, therefore, subsoiling along the edge of driveways is also needed.
- Avoid over-irrigating. Normal irrigation practices usually do not encourage Phytophthora blight except when leaks and puddles occur. Do not irrigate at night time when temperatures are above 70° F. Drip irrigation is a good way to provide water to a crop while avoiding creating potentially favorable conditions throughout the planting, including in bare-ground, direct-seeded pumpkin as shown below. Lay the drip tape a couple inches from the crown of plants because drip too close can create conditions favorable for crown rot.



- Do not irrigate from a pond that could contain water that drained from an infested field.
- Several fungicides have been developed with targeted activity for P. capsici and other oomycetes. Limited development of blight has been observed in plantings where these fungicides have been applied regularly, especially where a preventive spray program was used rather than waiting until symptoms were found. There also are several biopesticides labeled for application to soil before or at planting and to foliage. For current information see Fungicides for Managing Phytophthora Blight in Cucurbits and Other Vegetables.
- Scout fields for symptoms routinely, especially after major rain storms. Include any areas where water did not drain well and near the end of irrigation pipe. But also look elsewhere. Symptoms have been found in areas where least expected, including on the slope of high areas.
- When symptoms are localized in a small area of a field, disking the area is worthwhile. Begin with a border of healthy-appearing crop around the affected area.
- Do not discard cull fruit in the field, including fruit that are healthy but over-sized or over-ripe.
- Fruit that look healthy should be removed from infested fields as soon as possible and checked routinely for symptom development so that fruit developing symptoms after harvest can be discarded before the fungus spreads further. Asymptomatic affected fruit should develop symptoms within a week. It is especially important to harvest before rain. Growers have asked about disinfectants. None are registered for this use. Furthermore, applying a disinfectant to fruit will only kill Phytophthora spores on the fruit at the time; it will not stop the fungus if it has already started to infect the fruit and it will not affect spores that land on the fruit after treatment.
- Do not display pumpkin fruit for sale in a field where Phytophthora blight developed in previous years: healthy fruit have developed fruit rot in these situations even when the land had been planted to sod.
- Do not save seed from a field where Phytophthora blight occurred.
- Other practices that will improve water management will help control Phytophthora blight.
More information/prepared by:
Margaret Tuttle McGrath
Associate Professor Emeritus
Long Island Horticultural Research and Extension Center (LIHREC)
Plant Pathology and Plant-Microbe Biology Section
School of Integrative Plant Science
College of Agriculture and Life Sciences
Cornell University
mtm3@cornell.edu